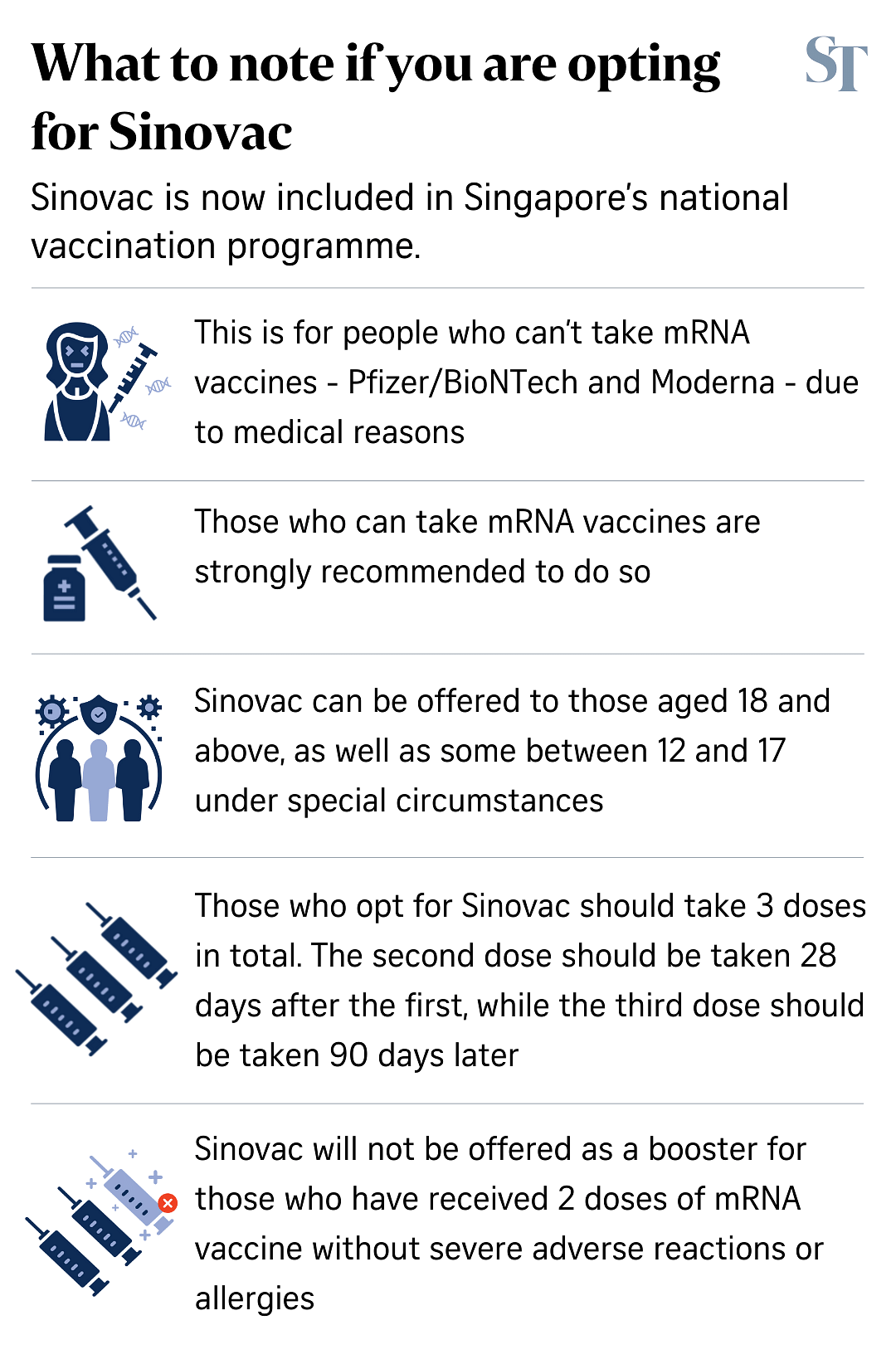
SINGAPORE - The Sinovac Covid-19 vaccine will be included in the national vaccination programme to cater to individuals aged 18 years and above who are unable or unwilling to take the Pfizer-BioNTech or Moderna mRNA vaccines.
The Ministry of Health said that three doses of the Sinovac vaccine will be required for a person to be considered fully vaccinated.
The second dose should be taken 28 days after the first dose, while the third dose should be taken 90 days after the second dose.
The Health Sciences Authority's (HSA) said it conducted a careful and thorough review taking into account the public health needs in Singapore of having non-mRNA vaccines as an option for individuals who are medically unsuitable to receive mRNA vaccines.
Here are some frequently asked questions on the Sinovac vaccine.
Q: What is the efficacy of Sinovac?
A: Vaccine efficacy is the degree to which a vaccine prevents a disease under controlled conditions in a clinical trial.
A study conducted in Brazil demonstrated vaccine efficacy of 51 per cent against non-Delta variants, which meets the threshold of 50 per cent set by the World Health Organisation (WHO) for Emergency Use Listing. This means that there is a 51 per cent reduction of symptomatic Covid-19 disease in a vaccinated group of people as compared with a similarly sized group of unvaccinated people.
The HSA's clinical review was based primarily on this data.
The authority also reviewed data from pre-clinical studies, clinical trials in human volunteers, manufacturing and quality controls, as well as supplemental data from a real-world effectiveness study in Chile.
The study in Chile involved more than 10 million participants aged 16 years and above.
As at May this year, Sinovac demonstrated vaccine effectiveness of 66 per cent against the Alpha and Gamma variants, according to the study.
The study also showed that Sinovac offered more than 86 per cent protection against other Covid-19 disease outcomes such as hospitalisation, admission to the intensive care unit and death.
There is a lack of data on the vaccine's protection against the Delta variant, as well as its effectiveness in immunocompromised patients and persons with co-morbidities such as diabetes, cardiovascular diseases and cancers.
Q: Why are three doses needed?
A: While Sinovac did not apply for Pandemic Special Access Route (PSAR) authorisation of a three-dose regimen, there is evidence that a third dose of Sinovac is needed at three to six months after the second dose to raise antibody levels, the Expert Committee on Covid-19 Vaccination said.
This is consistent with the WHO Strategic Advisory Group of Experts recommendation of a third dose for those aged 60 and above for the primary vaccination series.
There has been similar evidence that antibody levels from the Sinopharm vaccine also decline after two doses, and a third dose increases this substantially.
Individuals who opted for the Sinopharm vaccine as their primary series vaccination despite being eligible for the mRNA vaccines are strongly recommended to take one of the mRNA vaccines for the third dose of their primary series vaccination.
If they decline, they should take a third dose of the Sinopharm vaccine three months after the second dose to complete their primary vaccination series.
Q: How safe is Sinovac?
A: Based on data accrued from clinical trials to date, the safety profile of Sinovac is generally consistent with other registered vaccines used in immunisation against other diseases, the HSA said.
Some common side effects may include headache, injection site reaction, muscle pain and general discomfort after vaccination.
These symptoms are reactions generally associated with vaccinations and are expected as part of the body's natural response to build immunity against Covid-19. These side effects usually resolve on their own within a few days.
Q: What is the incidence rate of severe allergic reactions to Sinovac?
A: As at Monday (Oct 18) this year, the incidence of severe allergic reactions was 0.003 per cent of doses administered, the HSA said.
"As with all vaccines, there will be a small proportion of susceptible persons who experience severe allergic reactions upon vaccination," the authority added.
They include those with a history of anaphylaxis (or the rapid onset of severe allergic reactions), or hypersensitivity to the vaccine or its components.
In cases of severe allergic reactions, immediate medical attention should be sought.
People who develop anaphylaxis or severe allergic reactions to the first dose of the vaccine should also not be administered the second dose.
HSA said that due to insufficient data, it cannot make recommendations on use of the vaccine in pregnant women, severely immunocompromised people, people with co-morbidities and those under the age of 18.
Q: Why did HSA grant PSAR interim authorisation for the Sinovac vaccine?
A: HSA said the vaccine met the minimum technical requirements for use during a pandemic, given the urgent public health needs.
It added that it will continue to actively review evolving vaccine effectiveness and safety data to ensure that the benefits of the vaccine continue to outweigh the known risks.
The PSAR interim authorisation may also be terminated at any time; for example, if new data suggests that the benefits no longer outweigh the risks, the agency noted.
This article was edited for clarity.